Charcot shoulder neuroarthropathy



Clinical History
A 32-year-old man presented at the orthopaedics department for evaluation of a right shoulder deformity. History revealed that swelling was first noticed 9 months ago. Physical examination showed a painless, swollen right shoulder girdle without erythema. Range of motion of the joint was painless but severely restricted in all planes.
Imaging Findings
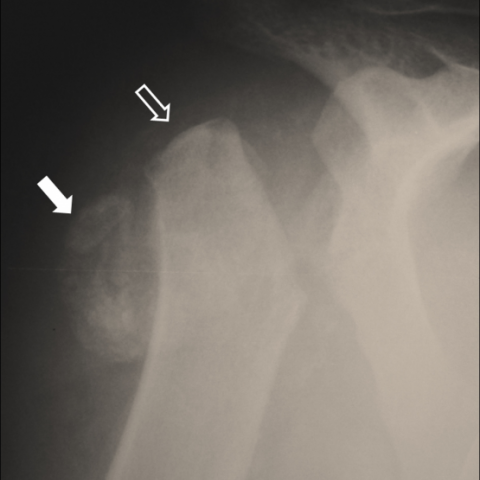
Laboratory examinations were within normal limits and the patient did not recall a recent or remote traumatic event. Plain X-ray (Fig. 1) showed a disorganised shoulder joint with evidence of humeral head resorption and accompanying soft tissue ossification. Differential diagnosis included inflammatory arthritis such as CPPD and gout, infectious processes such as septic arthritis and osteomyelitis and bone tumours. The patient remained asymptomatic with no clinical or laboratory evidence of inflammation or infection. A subsequent MDCT examination (Fig. 2-3) verified the presence of an amputated humeral head along with ossified debris in the subdeltoid-subacromial bursa and in the axillary pouch. The following MRI (Fig. 4) revealed significant synovial proliferation and thickening, joint and bursal effusion, intra-articular debris and no evidence of bone marrow oedema. Findings were inconclusive and the patient was referred for a CT guided biopsy (Fig. 5). During the procedure, the patient showed no painful reaction to multiple insertions of the needle, even in areas not previously infiltrated with lidocaine. Histopathologic examination was negative for malignancy. The patient’s unremarkable medical history in correlation with the clinical, laboratory, histopathologic and radiologic findings suggested the diagnosis of Charcot neuroarthropathy. An additional MRI of the spine (Fig. 6) verified the presence of syringomyelia as the underlying primary disorder.
Discussion
Neuropathic osteoarthropathy, also called neurotrophic joint, was first described by W. Musgrave in 1703. Later in 1868 J.M. Charcot was the first to report the findings and proposed an aetiology for the pathogenetic mechanism, thus the name Charcot neuroarthropathy-osteoarthropathy. Nowadays, two main theories for the pathophysiology of neuropathic arthropathy are proposed. The neurovascular theory suggests that an autonomous neuropathy leads to increased osseous blood perfusion which increases the osteoclastic activity and results in osteopenia. Subsequent pathologic fractures of the weakened bones cause severe destruction and deformity of joints. Loss of proprioception is believed to be the underlying condition in the neurotraumatic theory. The sensory deficiency leads to loss of protective sensation and finally the repetititive microtraumas lead to joint destruction. A third recent theory is supported today by some authors that combines the aforementioned pathogenetic mechanisms. Furthermore, advances in molecular biology have revealed that metabolic control of bone is influenced by the nervous system. A number of neurotransmitters like glutamate, calcitonin gene-related protein (CGRP), substance P, vasoactive intestinal peptide (VIP), pituitary adenylate cyclase activating polypeptide (PACAP), leptin, and catecholamines have been identified, establishing the role of the nervous tissue in regulating bone homeostasis and thus a molecular-based explanation might exist. Despite the unclear aetiopathogenetic mechanism various disorders have been implicated in neuropathic osteoarthropathy including diabetes mellitus, syringomyelia, tabes dorsalis (syphilis), spinal cord tumours, spinal cord injury, multiple sclerosis, poliomyelitis, leprosy and congenital or familial insensitivity to pain syndromes. Patients with diabetes mellitus are particularly prone to neuropathic arthropathy of the foot and ankle which is currently the more frequent location of the disease. In our case a syringomyelia-induced osteoarthropathy was encountered. To the best of our knowledge, although reports of shoulder Charcot arthropathy exist in the literature, disease demonstration with the use of volume rendering MDCT reformatting techniques has never been presented thoroughly. In conclusion, Charcot’s arthropathy may simulate aggressive disorders such as infection (osteomyelitis and/or septic arthritis) and tumor or inflammatory diseases with intraarticular deposits such as CPPD and gout. Thinking in retrospect, CT-guided biopsy could have been avoided after careful evaluation of all the clinical, physical, laboratory and initial imaging findings.
Differential Diagnosis List
Final Diagnosis
Charcot shoulder neuroarthropathy
Liscense
Figures
X-ray
CT

VRT Images



MRI


CT guided biopsy

MRI of the spine

Medical Imaging Analysis Report
1. Radiological Findings
Based on the provided X-ray, CT, and MRI images, there is significant joint structural destruction and deformity in the right shoulder joint region, presenting as follows:
- The articular surface of the shoulder joint is irregular, and the joint space is significantly narrowed or blurred. Localized bone destruction, fragmentation, and osteophyte-like growth are visible.
- The humeral head is markedly damaged in shape, with irregular fragments at the edges. The glenoid cavity also shows varying degrees of changes.
- Volumetric reconstruction on CT reveals a severe deformation of the joint contour, with substantial malalignment of the articular surface, appearing as a “collapse.”
- MRI shows mild soft tissue swelling around the joint, with no obvious signs of infection; partial tear or degenerative signals are visible in the shoulder’s soft tissue structures (e.g., rotator cuff).
- Combined with the cervical and upper thoracic MRI findings, syringomyelia (cavity formation in the spinal cord) is observed, suggesting pathological neurological impairment.
The overall imaging findings are disproportionate to the level of pain (the patient reports little or no pain), which further supports a diagnosis of “neuropathic arthropathy.”
2. Possible Diagnoses
Considering the patient’s medical history (no obvious acute trauma, no diabetes or other common causes, but with syringomyelia) and the radiological features, the likely diagnoses and differential diagnoses include:
- Neuropathic Arthropathy (Charcot Joint):
- Corresponding to the patient’s neurological deficits, commonly seen in diabetes, spinal cord injuries, leprosy, and syringomyelia.
- Radiographically characterized by progressive, destructive joint damage, with diminished or absent pain sensation.
- Infectious Joint Disease (e.g., osteomyelitis, septic arthritis):
- Usually presents with severe pain, redness, swelling, heat, and acute inflammatory signs. Blood tests often show elevated white blood cell counts and inflammatory markers.
- Imaging may also reveal bone destruction but typically with marked soft tissue swelling, abscess formation, or sinus tract formation.
- Crystal-Induced Arthropathy (e.g., gout, CPPD):
- Common in adults; acute attacks feature pronounced joint pain, redness, swelling, and heat, while chronic phases can cause joint damage.
- Typical imaging findings include local soft tissue swelling and characteristic crystal deposits.
- Tumors or Tumor-Like Lesions:
- Includes osteosarcoma, metastatic lesions, or other forms of local neoplastic invasions.
- Radiographically, they may present as eccentric lytic or sclerotic lesions, or as localized soft tissue masses.
Among these differentials, the painless joint destruction and history of syringomyelia most strongly support neuropathic arthropathy.
3. Final Diagnosis
Considering the above radiological features (severe and painless joint destruction), the patient’s clinical presentation (long-term painless swelling and restricted motion), and the confirmed history of syringomyelia, the diagnosis is: “Neuropathic Shoulder Arthropathy (Charcot Joint) Caused by Syringomyelia.”
Current clinical, imaging, and pathological evidence strongly supports this diagnosis. Further procedures such as biopsy may be cautiously considered or avoided if not strictly necessary.
4. Treatment Plan and Rehabilitation Program
4.1 Treatment Strategies
- Conservative Treatment:
- Primarily aimed at preventing further joint destruction. When needed, a brace or shoulder sling may be used for protective immobilization.
- Control and improve the progression of the underlying disease (e.g., syringomyelia) responsible for the neuropathic changes.
- For patients with mild joint pain, NSAIDs or analgesics may be considered to relieve discomfort.
- Surgical Treatment:
- In cases of severe deformity or impaired daily functioning, joint fusion or joint replacement surgery could be evaluated. However, surgery should be approached with caution in the context of neuropathic arthropathy due to higher risks.
- Surgical indications depend on functional needs, pain severity, and the stability of neurological deficits.
- Prevention of Complications:
- Prevent secondary skin breakdown, exacerbation of joint instability, or periarticular fractures.
- If there is any risk of infection, address potential infectious sources promptly.
4.2 Rehabilitation and Exercise Prescription
The goal of rehabilitation is to maintain or restore shoulder joint function as much as possible while preventing further damage. An individualized program can follow the FITT-VP principles (Frequency, Intensity, Time, Type, Progression, Volume):
- Phase 1 (Acute/Subacute Protection Stage)
- Frequency: 3–5 times per week, short sessions at low intensity.
- Intensity: Initiate range-of-motion training with passive or assisted movements, avoiding excessive stretching or twisting.
- Time: 5–10 minutes per session, gradually increasing over time.
- Type: Joint flexibility exercises and mild isometric contractions of the shoulder muscles.
- Precautions: Use braces or other supports for joint protection and monitor the patient’s subjective discomfort.
- Phase 2 (Functional Recovery and Strengthening Stage)
- Frequency: 3–4 times per week; the duration of sessions can be extended as tolerated.
- Intensity: Gradually increase the range of motion and muscle strength exercises while ensuring stability.
- Time: 15–20 minutes per session or adjusted according to tolerance.
- Type: Active range-of-motion training; shoulder muscle exercises using elastic bands or light weights, combined with shoulder girdle stabilization exercises.
- Progression: Once a certain intensity is well tolerated without undue discomfort, resistance may be gradually increased.
- Phase 3 (Maintenance and Re-Injury Prevention Stage)
- Frequency: 2–3 times per week, continued over the long term.
- Intensity: Maintain moderate intensity without striving for heavy loads.
- Time: 20–30 minutes per session, focusing on precise movements and control.
- Type: Stability and coordination training, such as closed-chain exercises and shoulder coordination drills.
- Progression: Depending on joint condition and functional needs, maintain or slightly increase training volume.
During the entire rehabilitation process, carefully monitor for changes in shoulder pain or swelling. If any acute discomfort or sudden functional decline occurs, seek medical attention promptly.
5. Disclaimer
This report provides an advisory analysis based on the current medical history and imaging data. It does not replace face-to-face consultation or a professional physician’s diagnosis and treatment plan. All medical decisions should be made in line with the patient’s actual condition and under the guidance of qualified healthcare professionals.
Human Doctor Final Diagnosis
Charcot shoulder neuroarthropathy