Skeletal muscle metastasis from lung adenocarcinoma


Clinical History
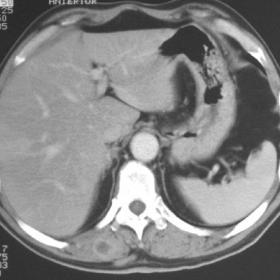
Bilateral masses in erector spinae muscles showing contrast enhancement on CT.
Imaging Findings
A small subcutaneous nodule in the substernal region was noted during the examination of this patient who presented with recurrent abdominal pain. After a negative abdominal US, CT examination was performed to elucidate the symptoms.
The CT revealed multiple contrast enhancing irregular lesions bilaterally in the erector spinae muscles, their features suggesting inflammation, parasitic infection or primary/metastatic muscular neoplasms. Two small subcutaneous nodules with soft tissue density were also seen, one in the right upper abdominal quadrant, the other in the substernal region. US guided biopsy of the muscle nodules was performed, as well as surgical excision of the two subcutaneous masses. Pathological examination revealed all masses to be metastasis from lung adenocarcinoma. Subsequent chest CT and bronchoscopy were negative.
Another CT examination at 30 days showed a small right-sided posterior lung mass, which at 2 months from the original diagnosis, after a course of chemotherapy, showed extension to the adjacent rib and muscle. CT-guided biopsy confirmed bronchial adenocarcinoma.
Discussion
Large autopsy studies report metastasis from lung cancer in nearly every organ system of the body. Frequent intrathoracic sites of lung cancer spread include hilar and mediastinal lymph nodes, pleura, diaphragm, chest wall, and pericardium, while common extrathoracic sites are liver, adrenal glands, bone, bone marrow, kidney and central nervous system. Less common sites of metastasis include the gastrointestinal tract, pancreas, thyroid, spleen, pituitary, abdominal lymph nodes, skin and oral cavity. Haematogeneous skeletal muscle metastasis from lung cancer is an extremely rare finding, while direct invasion of skeletal muscle in the chest wall and trunk is common in lung and other cancers. It must be remembered that primary tumours in skeletal muscle are more common than secondary tumours (1).
Metastatic patterns are not random and appear to be dependent on properties unique to the tumour cell and certain organs (2). These patterns have been postulated to result either from variations in the vascular anatomy of certain organs or from specific chemometabolic characteristics favouring the arrest and growth of tumour cells. Several factors critical to the establishment of metastases have been identified, and include: detachment of tumour cells, arrest of circulating tumour cells in vascular endothelium at specific sites, escape into the tissues and angiogenesis to permit the growth of a metastatic implant.
The reason for the rarity of metastatic tumours in skeletal muscle is unclear, but could be related to factors such as blood flow, metabolism, and high tissue pressure. Irrespective of blood flow, skeletal muscle may be a poor milieu for tumour cells and this may be related to lactic acid metabolism.
Haematogeneous skeletal muscle metastases should be suspected in cancer patients with pain in the location of larger skeletal muscles with negative radiological or radionuclide evaluations for osseous metastasis. Painless subcutaneous metastases are very common, and are easily recognised by physical examination to be superficial; they may be better felt when the underlying muscles are actively contracted. In contrast, skeletal muscle metastases are uncommon, deep in location, and painful and it may be not possible to distinguish them clinically in some patients.
This unusual site of metastasis can be localised with CT or ultrasonography in selected patients and confirmed by bedside thin needle aspiration. Identification of malignant cells by cytology using standard criteria will exclude more common causes of pain and swelling, such as infection, haematoma, or a ruptured muscle.
The optimal treatment of skeletal muscle metastasis is unknown as the prognosis is poor and there are few reports in the medical literature of surgical excision or radiation therapy of the involved muscles (3).
Differential Diagnosis List
Final Diagnosis
Skeletal muscle metastasis from lung cancer
Liscense
Figures
Muscular metastasis from lung adenocarcinoma



Imaging Findings
1. Bilateral block-like lesions can be seen in the erector spinae muscles on chest and abdominal CT images, with relatively clear boundaries, appearing relatively round or irregular in shape, and showing significantly increased density upon enhancement.
2. No obvious bone destruction is seen at the lesion level, but there is infiltration or edema of varying degrees in the surrounding soft tissues.
3. No obvious localized lesions in the liver or other abdominal organs; however, correlation with the patient’s clinical presentation and other imaging sequences is needed for comprehensive evaluation.
4. Compared with the surrounding muscle, the density/signal of the lesion differs significantly, pointing to a space-occupying lesion with enhancement.
Possible Diagnoses
Based on the patient’s history (suspected or confirmed lung cancer) and imaging features, the following diagnoses are worth considering:
1. Lung cancer metastasis to skeletal muscle: Hematogenous spread of lung cancer to skeletal muscle is rare, but previous literature has confirmed its possibility, especially in the chest wall, back, or other areas adjacent to the lungs or with rich blood supply.
2. Primary skeletal muscle tumor (e.g., sarcoma): It is necessary to exclude primary muscle tumors. Although the overall incidence is relatively low, similar space-occupying lesions with enhancement can appear locally.
3. Inflammatory or infectious lesions: For example, muscle abscess, specific or nonspecific infections (tuberculosis, fungal, etc.) can also present as space-occupying lesions on CT, but typically are accompanied by more obvious peripheral inflammatory edema and relevant clinical manifestations (such as fever, increased pain, and elevated infection markers).
4. Benign tumor or pseudotumor: Such as hemangioma, lipoma, or proliferative lesions. The pattern of enhancement and clinical context differ, requiring evaluation of the internal nature of the lesion.
Final Diagnosis
Considering the patient’s age (50 years old), male, prior history of lung cancer (or high suspicion), bilateral symmetrical lesions in the erector spinae, and marked enhancement characteristics, the most likely diagnosis is hematogenous metastasis of lung cancer to the erector spinae.
Since skeletal muscle metastasis is relatively rare, if both clinical and imaging findings are highly suspicious, an ultrasound- or CT-guided biopsy may be performed to confirm the pathology.
Treatment Plan and Rehabilitation
1. Overview of Treatment Strategy:
- Systemic Treatment: If confirmed as metastatic lung cancer, collaborate with oncology to evaluate the appropriate systemic therapy, including chemotherapy, immunotherapy, or targeted therapy.
- Local Treatment: Depending on lesion size, symptoms, and the impact on surrounding structures, local radiotherapy or surgical resection can be considered. Since muscle metastasis often indicates a later stage of disease, radical surgery may have limited curative value; the main goals may be symptom relief, pain control, or prevention of pathological damage.
- Supportive Treatment: Includes pain management, nutritional support, and psychological support. If functional impairment exists (e.g., restricted mobility or pain), symptomatic rehabilitation therapy can be instituted.
2. Rehabilitation/Exercise Prescription Suggestions:
- General Principles: Use a gradual, individualized approach to develop an exercise program, if the patient’s condition permits. If symptoms are severe or active chemotherapy/radiotherapy is ongoing, reduce exercise intensity appropriately and monitor for complications.
- Types of Exercise: Begin with low-intensity aerobic exercises, such as walking or low-speed stationary cycling, for about 10–15 minutes each time, 3 times per week. Gradually increase to 20–30 minutes, 3–5 times per week.
- Strength Training: If pain is tolerable and muscle function remains sufficient, moderate resistance training (e.g., resistance bands, light dumbbells) can be introduced. Focus on muscle groups not affected by the lesions to avoid aggravating pain or causing injury to the involved area.
- Monitoring and Adjustment: Assess pain and fatigue levels before and after each session (using a numeric pain scale or Borg scale). If pain intensifies or fatigue worsens significantly, reduce the exercise load or temporarily stop. Consult rehabilitation or sports medicine specialists when needed.
- Progressive Advancement (FITT-VP Principle):
• Frequency: Increase from 2–3 sessions per week to 3–5 sessions per week over time.
• Intensity: Start with low intensity (e.g., maintaining heart rate at 40%–50% of maximum), increasing gradually if tolerated.
• Time: Initially 10–15 minutes. Every 1–2 weeks, increase by 5–10 minutes, as appropriate.
• Type: Begin with aerobic activities (walking, stationary cycling) and progressively add low-intensity anaerobic/resistance training.
• Volume & Progression: Overall exercise volume is determined by rehabilitation goals and disease status, with re-evaluation and minor adjustments every 2–4 weeks.
Precautions:
- In cases of osteoporosis, muscle fatigue, or compromised cardiopulmonary function, monitor vital signs closely during exercise to prevent overload.
- If there is marked pain or risk of pathological fracture, specialized exercise under medical or rehabilitation supervision is recommended to avoid new injuries.
Disclaimer: This report is presented solely as a reference-based analysis of existing imaging and clinical information. It does not replace in-person consultation or professional medical advice. If any doubts arise or symptoms worsen, please seek medical attention promptly.
Human Doctor Final Diagnosis
Skeletal muscle metastasis from lung cancer