Kimura’s disease in the groin of a middle-aged woman



Clinical History
A 42-year-old female was referred to our hospital with an approximately ten-year-history of a slowly growing right inguinal lump. Physical examination revealed a solitary mass measuring 7×5 cm with a normal overlying skin. She had slight tenderness over the right inguinal region. Blood investigation showed peripheral eosinophilia (49% of white blood cell count: 12,500/μL).
Imaging Findings
Computed tomography (CT) revealed a subcutaneous isodense mass with an irregular margin and regional lymphadenopathy in the right groin (Fig. 1). In magnetic resonance imaging (MRI), the signal of the mass was isointense to the muscle on T1-weighted images, and hyperintense foci corresponding to the fat and signal voids were identified in the lesion (Fig. 2). On T2-weighted images, the lesion was heterogeneous and also contained hyperintense foci and signal voids (Fig. 3). Coronal T1-weighted images showed hyperintense nodular components and irregular shaped isointense areas within the mass lesion (Fig. 4a), and the signals of the nodular components and the enlarged lymph nodes were intensely enhanced on fat-suppressed postcontrast images (Fig. 4b).
Discussion
Kimura’s disease (KD) is a rare, benign, chronic inflammatory disorder of unknown etiology, which was systemically analyzed by Kimura et al. in 1948 [1,2]. The lesion is characterized by consistent histologic features such as follicular hyperplasia, eosinophilic infiltrates and proliferation of postcapillary venules [3].
Clinical Perspective
Kimura’s disease typically presents as a painless subcutaneous mass in the head and neck, frequently associated with regional lymphadenopathy and/or a salivary gland involvement, but rarely involves the other anatomical sites such as the trunk and extremities [4]. It is characterized by elevated serum Immunoglobulin E (IgE) and peripheral blood eosinophilia [3]. Because the lesion is easily invasive and accompanied by enlarged lymph nodes, differentiation from acute inflammatory lesions and malignancy becomes important issue. Preoperative diagnosis is even more difficult when the lesion is located in atypical sites, as in this case. Although pathological evidence is essential for the definite diagnosis of KD, CT and MRI images are also useful in identifying the extent of the disease and suggesting a diagnosis.
Imaging Perspective
Although the imaging findings of KD may vary or not specific, the lesions are usually presented as ill-defined infiltrative masses in the subcutaneous tissue associated with lymphadenopathy. Some previous studies have suggested that the subcutaneous mass in KD is identified as a hypervascular lesion with signal voids and homogenous enhancement after gadolinium injection [5,6]. An additional feature is the presence of irregular and thick strands of hyperintense signals corresponding to fatty tissue within the lesion, which implies an infiltrative nature of the inflammatory disorder [7]. When a subcutaneous lesion with these findings of flow voids, fat component (T1 hyperintense), and lymphadenopathy is encountered, elevated eosinophils and IgE should be confirmed. The final diagnosis is made by pathological evidence.
Outcome
Although a standard therapy of KD has not yet been established, treatment options of the disease include surgical resection, regional or systemic steroid therapy, immunosuppressive agents (e.g., cyclosporine, omalizumab, or imatinib), cytotoxic therapy and radiation [4,8,9]. In our case, the patient had a good clinical response with corticosteroid therapy and her inguinal mass and regional lymph nodes reduced in size and signal intensity on T2-weighted image. It is assumed that the anti-inflammatory effect reduced the infiltration of lymphocytes and eosinophils and induced fibrosis of the stroma. An excision was performed after corticosteroid therapy, and the diagnosis was pathologically confirmed.
Take Home Message / Teaching Points
When cases of ill-defined subcutaneous mass with flow voids, fat component (T1 hyperintense), and lymphadenopathy are encountered, Kimura's disease should be taken into consideration.
Final Diagnosis
Kimura’s disease
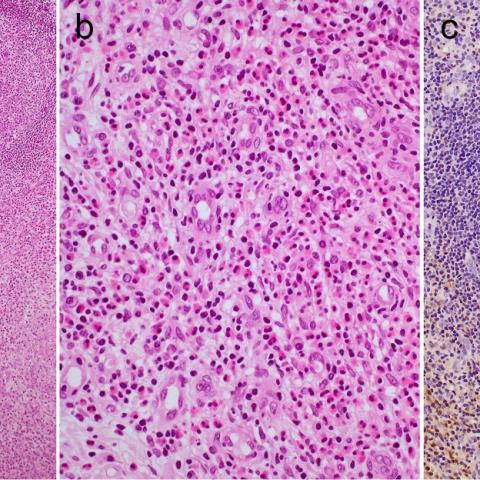
Skin biopsy was performed to obtain critical information on the diagnosis. Histologically, the lesion located subcutaneously was made up by multiple well-defined or enlarged lymphoid follicles with prominent germinal centers surrounded by hypercellular interfollicular areas containing many eosinophils and postcapillary venules (Fig. 5a & b). The germinal centers also contained eosinophils, and a granulomatous area with eosinophilic necrosis was present. Immunohistochemically, a reticular staining pattern of IgE deposition was seen in the germinal centers (Fig. 5c).
All patient data have been completely anonymized throughout the entire manuscript and related files.
Differential Diagnosis List
Final Diagnosis
Kimura’s disease
Liscense
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Figures




Imaging Findings
A relatively ill-defined soft tissue mass is observed in the right inguinal region. Plain CT shows a mildly increased density of the soft tissue, along with enlargement of nearby lymph nodes. On MRI T1-weighted images, parts of the lesion appear as areas of high signal intensity; on T2-weighted images, the lesion shows a relatively high signal. Focal flow-void signals (suggesting abundant vasculature) and mixed fatty components are also noted. Overall, the lesion demonstrates an infiltrative pattern and is poorly delineated from surrounding tissues.
Clinical examination reveals a marked increase in peripheral blood eosinophils (up to 49%), and the patient reports that the mass has been present for a long period, growing slowly, with a good response to steroid therapy.
Possible Diagnoses
- Kimura’s Disease: Commonly presents as superficial soft tissue masses in the head and neck region or other areas, often accompanied by regional lymph node enlargement, peripheral eosinophilia, and elevated IgE levels. Histologically, there is lymphoid follicular hyperplasia with prominent eosinophilic infiltration. These features match both the imaging and laboratory findings in this case.
- Lymphoma: Can cause soft tissue or superficial lymph node enlargement but often involves more extensive lymph nodes. Abnormal peripheral blood tests can vary and are not necessarily dominated by eosinophilia. Pathological examination helps to differentiate from the present case.
- Localized infection or parasitic disease: For instance, filariasis can lead to regional lymph node or soft tissue swelling with accompanying eosinophilia. However, the imaging findings here are not entirely consistent with secondary edema or abscess, so pathology or serological tests are needed for differentiation.
Final Diagnosis
Based on the patient’s long-standing right inguinal mass, imaging findings (diffuse infiltrative soft tissue lesion, flow-void signal, enlarged lymph nodes, and mixed fatty components), significant peripheral eosinophilia, and good response to corticosteroids, the final diagnosis is Kimura’s Disease. Histopathologic examination confirms lymphoid follicular hyperplasia with abundant eosinophilic infiltration, consistent with the disease characteristics.
Treatment and Rehabilitation Plan
Treatment Strategy:
- Pharmacotherapy: Initial treatment may involve systemic corticosteroids (e.g., prednisone) to reduce the inflammatory response and eosinophilic infiltration. In recurrent or refractory cases, immunosuppressants (e.g., cyclosporine) or monoclonal antibodies (e.g., omalizumab) may be considered.
- Surgical Intervention: For large local masses or those impacting function or appearance, surgical excision may be performed after inflammation subsides or stabilizes. This also provides a pathological diagnosis and removal of the lesion.
- Radiotherapy: Rarely used in clinical practice, mainly reserved for recurrent cases or patients unable to tolerate other treatments.
Rehabilitation and Exercise Prescription:
After controlling inflammation and achieving disease stability with medication, patients can gradually begin appropriate exercise to enhance physical fitness, promote circulatory function, and support immune balance. Adherence to the FITT-VP Principle (Frequency, Intensity, Time, Type, Progression, Volume) is recommended:
- Frequency (F): 3–5 sessions per week are suitable. Individuals without significant cardiopulmonary disease may gradually increase to daily light activities.
- Intensity (I): Focus on low to moderate intensity. If experiencing fatigue or joint pain, start with low intensity to avoid overexertion.
- Time (T): Begin with 15–20 minutes per session at the initial stage and gradually increase to 30–45 minutes, depending on tolerance.
- Type (T): Emphasize safe and easily performed aerobic exercises, such as walking, stationary cycling, or swimming. Incorporate simple strength training (e.g., resistance bands or light dumbbells).
- Progression (P) & Volume (V): As the condition improves and muscle strength increases, reevaluate every 2–4 weeks to make slight increments in exercise volume. If significant fatigue, pain, or noticeable enlargement of the local mass occurs, reduce or pause activity and consult a specialist promptly.
During rehabilitation, close monitoring of the local mass is essential. If the mass shows significant enlargement, pain, or signs of fever, a prompt medical evaluation is advised to rule out recurrence or infection.
Disclaimer:
This report is based on provided clinical and imaging data for reference and does not substitute an in-person evaluation or individualized guidance from a qualified physician. If any questions arise or there is a change in condition, please seek advice from a specialist without delay.
Human Doctor Final Diagnosis
Kimura’s disease